Regulation
EPI 425/2016

CE Marking for Protective Gloves
Protective gloves are Personal Protective Equipment or PPE.
PPE products used to be certified according to European Directive 89/686/EC.
The visible indication of compliance was affixed with the CE mark, serving to inform authorities and users that the PPE complied with mandatory legislation.
New PPE Regulation (EU) 2016/425
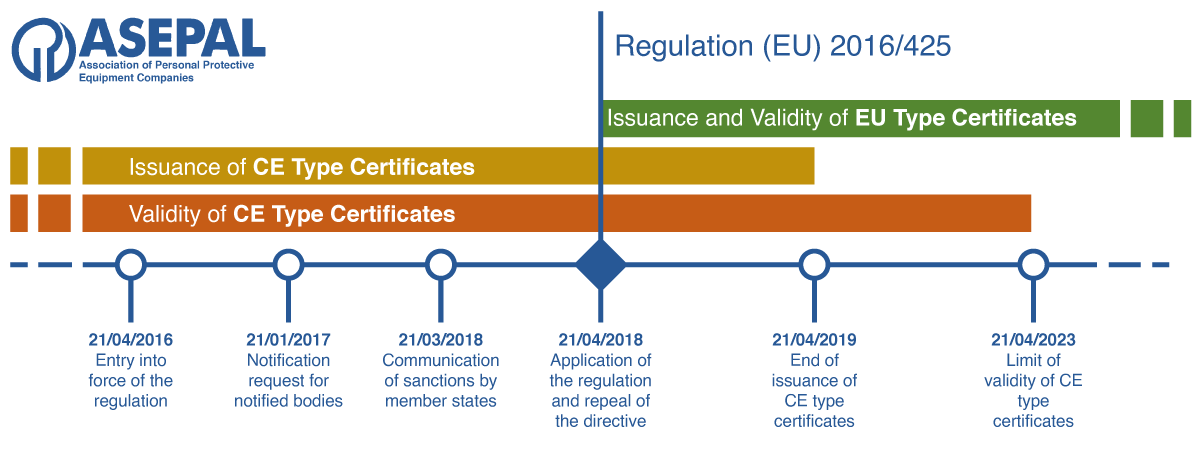
As of April 21, 2018, Directive 89/686/EEC was replaced by the new Regulation (EU) 2016/425 of the European Parliament and of the Council, dated March 9, 2016, on Personal Protective Equipment. This regulation slightly amends the scope and risk categorization of products and also clarifies the documentary obligations of economic operators.
Products bearing the CE mark according to Regulation (EU) 2016/425 began to be marketed from April 21, 2018.
Since April 21, 2019, all marketed products must be certified and CE marked under Regulation (EU) 2016/425, and starting from April 2023, no product that does not comply with it can be marketed.
The transition from the PPE Directive to the PPE Regulation brought about several changes, including changes in product categorization related to associated risk.
Product categorization change related to a related risk. Changes in the classification of certain product categories.
CE Declaration of Conformity, which will be provided with each product (or via a web link).
Validity/expiry date of 5 years for new EU certificates.
Increased obligations for “economic operators,” i.e., the entire supply chain, including manufacturers, importers, and distributors. They are all required to know the products they market and ensure their safety, taking responsibility for their sale.
Categories Classification
Category of PPE
Category I
Category II
Category III
Category Description
Simple PPE (designed to protect users from minimal risks).
Intermediate PPE (not included in Category I or Category III).
Complex PPE (which can cause very serious consequences, such as death or irreversible damage to health).
Activity
Product introduction into the market: self-declaration by manufacturers.
Initial product approval.
Ongoing monitoring through testing or manufacturing audits.
All our Personal Protective Equipment (PPE) falls under Category III.
EN 21-420 Standard
(PPE Legislation)
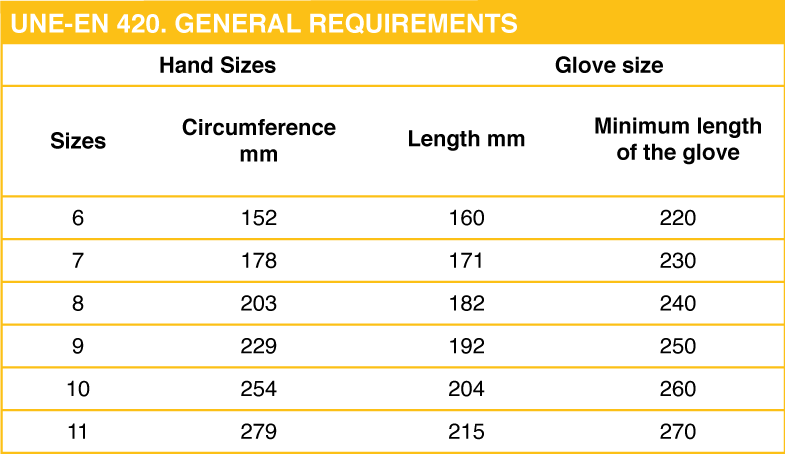
This standard sets out the general requirements and appropriate test procedures for glove construction and design.
Each protective glove will be marked with the following information:
- Name, brand, or other means of manufacturer identification or their authorized representative.
- Glove designation and size.
- If necessary, marking related to the expiration date.
- When the glove complies with one or more European standards, the appropriate pictogram for that standard will be used.
EN 374 Standard
EN 374-1:2016 (PPE Legislation – Chemical Risks)
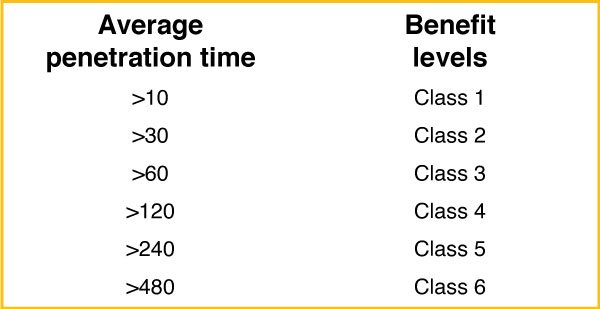
Protective gloves against chemical products are based on three test methods: penetration, permeation, and degradation.
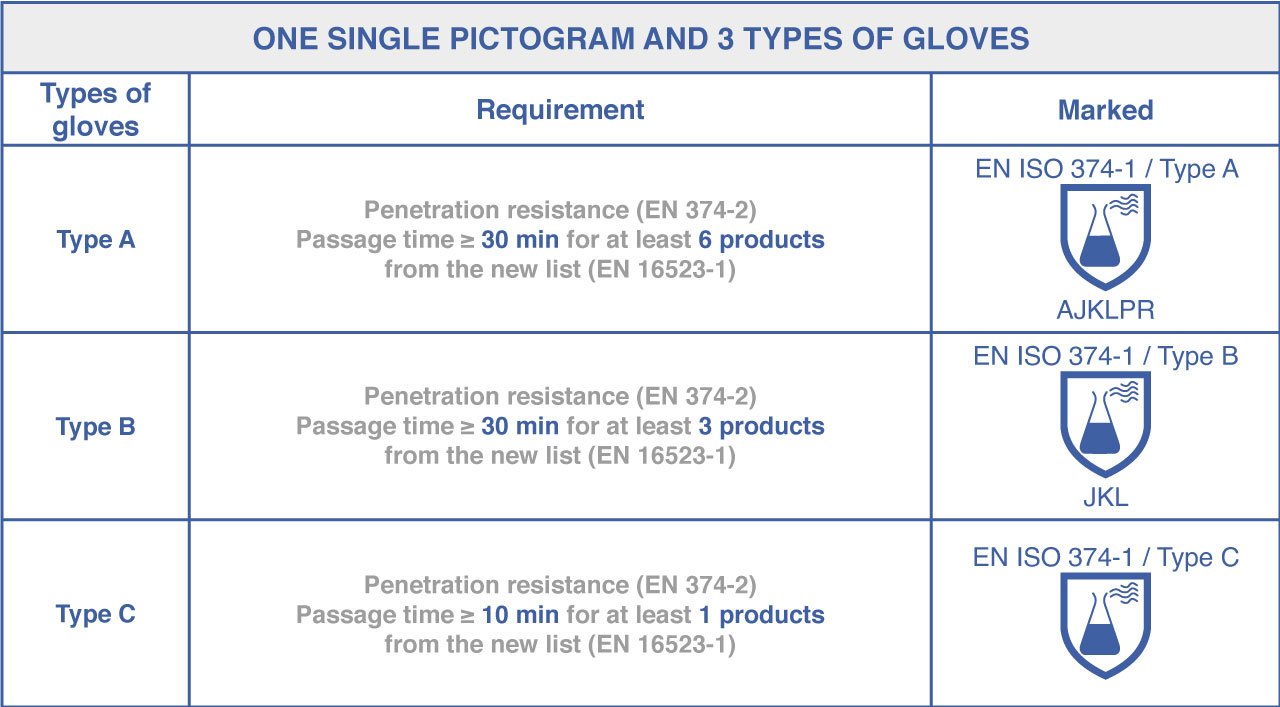
Based on this standard, all our gloves belong to Category B.
EN 374-5:2016 (PPE Legislation – Chemical Risks)
Protective gloves against chemical products and hazardous microorganisms, bacteria, fungi, and viruses. Gloves must pass the penetration resistance test according to standard EN 374-2:2014. The possibility of claiming protection against viruses has been added, if the glove is subjected to ISO 16604:2004 (Method B) testing.

For gloves offering protection against bacteria and fungi.

For gloves protecting against bacteria, fungi, and viruses.
For gloves offering protection against bacteria and fungi.
List of Chemicals: